Have you ever wondered what makes a body hot? The answer may be simpler than it seems.
The temperature of an object increases when the molecules that make up that object move faster.
Thermal energy
This is the energy possessed by an object or system as a result of the movement of particles within the object or system.
Thermal energy is one of the various types of energy, where "energy" can be defined as "the ability to do work."
Job
This is the movement of an object due to an applied force.
A system is simply a collection of objects within some boundary. Therefore, thermal energy can be described as the ability of something to do work through the movement of its particles. Since thermal energy is caused by the movement of particles, it is a type of kinetic energy that is the energy of motion. Heat energy causes something to have an internal temperature, and this temperature can be measured in degrees Celsius or Fahrenheit on a thermometer, for example.
The faster particles move within an object or system, the higher the recorded temperature.
Component structure
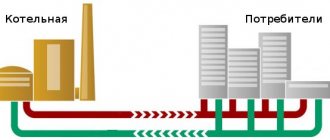
In order to heat the water that enters a large city house, it is necessary to expend heat and electricity . Electricity is used by all kinds of auxiliary mechanisms - pumps, regulators, etc. The heat is used to heat a certain flow rate to the required temperature.
The sum of these two components is considered as thermal energy, for which residents must pay.
At the same time, you need to understand that not all of the invested energy reaches the end consumer; some of it is lost along the way - the water cools down in the pipes, there are leaks and similar troubles. But the consumer pays for all the energy expended.
As a result, the citizen pays all the supplier’s costs for heating water and delivering it to the apartment.
Where can I find the line?
The invoice-receipt for payment of housing and communal services (HCS) is drawn up in the form of a table. The left column lists the types of services, and their characteristics are given horizontally to the right. The second line in the table from the top is called “Hot water supply.
| Accrued | Unit change | Tariff\Size boards | Col. | Accrued | Benefit | To pay |
| Cold water supply | ||||||
| Hot water supply | M3 | 198,19 | 1,80 | 356,74 | 89,18 | 267,56 |
| Water disposal |
Often in an apartment building, residents see separately in their document how much they need to pay for the consumption (measured in cubic meters) of hot water (with characteristics that comply with the regulations) and the cost of heating their apartment. The payment for thermal energy is stated in these two lines of the receipt.
Gigacalorie - what is it?
09.09.2017Gigacalorie - what is it?
Education Colleges and Universities
Av. Yulia Shamshurina August 18, 2017
Most people look forward to the New Year during the frosty winter months, and least of all to heating bills. They are especially disliked by residents of apartment buildings, who themselves do not have the ability to control the amount of incoming heat, and often the bills for it turn out to be simply fantastic. In most cases, in such documents the unit of measurement is Gcal, which stands for “gigacalorie”.
Let's find out what it is, how to calculate gigacalories and convert to other units.
What is a calorie?
Proponents of a healthy diet or those who closely monitor their weight are familiar with the concept of a calorie. This word means the amount of energy obtained as a result of the body processing the food eaten, which must be used, otherwise the person will begin to gain weight.
Paradoxically, the same value is used to measure the amount of thermal energy used to heat rooms. In physics, it is generally accepted that one calorie is the amount of energy required to heat one gram of H2O by 1 °C at standard atmospheric pressure (101,325 Pa).
As an abbreviation, this value is designated as “cal”, or in English cal.
In the metric system of measurements, the equivalent of a calorie is the joule. So, 1 cal = 4.2 J.
The importance of calories for human life
In addition to developing various weight loss diets, this unit is used to measure energy, work and heat. In this regard, such concepts as “calorie content” are common - that is, the heat of combustible fuel.
In most developed countries, when calculating heating, people no longer pay for the number of cubic meters of gas consumed (if it is gas), but precisely for its calorie content. In other words, the consumer pays for the quality of the fuel used: the higher it is, the less gas will have to be used for heating. This practice reduces the possibility of diluting the substance used with other, cheaper and less calorific compounds.
What is a gigacalorie and how many calories are in it?
As is clear from the definition, the size of 1 calorie is small. For this reason, it is not used to calculate large quantities, especially in the energy sector. Instead, the concept of gigacalorie is used. This is a value equal to 109 calories, and it is written as the abbreviation “Gcal”. It turns out that there are one billion calories in one gigacalorie.
In addition to this value, a slightly smaller one is sometimes used - Kcal (kilocalorie). It holds 1000 cal. Thus, we can consider that one gigacalorie is a million kilocalories. It's worth keeping in mind that sometimes a kilocalorie is written simply as "feces." Because of this, confusion arises, and some sources indicate that there are 1,000,000 calories in 1 Gcal, although in reality we are talking about 1,000,000 Kcal.
Hecacalorie and gigacalorie
In energy, in most cases the Gcal is used as a unit of measurement, but it is often confused with such a concept as “hecacalorie” (also known as hectocalorie). In this regard, the abbreviation “Gcal” is interpreted by some people as “hecacalorie” or “hectocalorie”. However, this is wrong. In fact, the above-mentioned units of measurement do not exist, and their use in speech is the result of illiteracy, and nothing more.
Gigacalorie and gigacalorie/hour: what is the difference
In addition to the fictitious value in question, receipts sometimes contain an abbreviation such as “Gcal/hour.” What does it mean and how does it differ from the usual gigacalorie? This unit of measurement shows how much energy was used in one hour. While just a gigacalorie is a measurement of heat consumed over an indefinite period of time. It depends only on the consumer what time frame will be indicated in this category.
The reduction Gcal/m3 is much less common. It means how many gigacalories need to be used to heat one cubic meter of a substance. Gigacalorie formula Having considered the definition of the value being studied, it is worth finally finding out how to calculate how many gigacalories are used to heat a room during the heating season. For especially lazy people on the Internet, there are a lot of online resources where specially programmed calculators are presented. All you have to do is enter your numerical data - and they themselves will calculate the number of gigacalories consumed. However, it would be nice to be able to do this yourself.
There are several formula options for this. The simplest and most understandable among them is the following: Thermal energy (Gcal/hour) = (M1 x (T1-Txv)) - (M2 x (T2-Txv)) / 1000, where: M1 is the mass of the heat-transferring substance that is supplied through the pipeline . Measured in tons. M2 is the mass of the heat transfer substance returning through the pipeline. T1 is the temperature of the coolant in the supply pipeline, measured in Celsius. T2 is the temperature of the coolant returning back. Тхв is the temperature of the cold source (water). Usually equal to five degrees Celsius, since this is the minimum temperature of water in the pipeline.
Why do housing and communal services overestimate the amount of energy spent when paying for heating? When making your own calculations, it is worth noting that housing and communal services slightly overestimate the standards for thermal energy consumption. The idea that they are trying to earn extra money from this is wrong. After all, the cost of 1 Gcal already includes maintenance, salaries, taxes, and additional profit. This “surcharge” is due to the fact that when hot liquid is transported through a pipeline in the cold season, it tends to cool down, that is, inevitable heat loss occurs. In numbers it looks like this. According to regulations, the temperature of water in heating pipes must be at least +55 °C.
And if we take into account that the minimum temperature of water in power systems is +5 °C, then it must be heated by 50 degrees. It turns out that 0.05 Gcal is used for each cubic meter.
However, in order to compensate for heat loss, this coefficient is inflated to 0.059 Gcal. Converting Gcal to kW/hour Thermal energy can be measured in various units, but in official documentation from housing and communal services it is calculated in Gcal. Therefore, it is worth knowing how to convert other units to gigacalories.
The easiest way to do this is when the relationships between these quantities are known. For example, it is worth considering watts (W), in which the energy output of most boilers or heaters is measured. Before considering the conversion to this Gcal value, it is worth remembering that, like a calorie, a watt is small. Therefore, kW (1 kilowatt equals 1000 watts) or mW (1 megawatt equals 1000,000 watts) are more often used.
In addition, it is important to remember that power is measured in W (kW, mW), but kW/h (kilowatt-hours) is used to calculate the amount of electricity consumed/produced. In this regard, it is not the conversion of gigacalories to kilowatts that is considered, but the conversion of Gcal to kWh. How to do this? In order not to suffer with formulas, it is worth remembering the “magic” number 1163. This is exactly how many kilowatts of energy must be spent in an hour to get one gigacalorie.
In practice, when converting from one unit of measurement to another, you simply need to multiply the amount of Gcal by 1163. For example, let's convert into kW/hour 0.05 Gcal required to heat one cubic meter of water by 50 °C. It turns out: 0.05 x 1163 = 58.15 kW/hour. These calculations will especially help those who are thinking about changing gas heating to more environmentally friendly and economical electric heating. If we are talking about huge volumes, we can convert it not into kilowatts, but into megawatts.
In this case, you need to multiply not by 1163, but by 1.163, since 1 mW = 1000 kW. Or simply divide the result obtained in kilowatts by a thousand. Conversion to Gcal Sometimes it is necessary to carry out the reverse process, that is, to calculate how many Gcal are contained in one kW/hour. When converting to gigacalories, the number of kilowatt-hours must be multiplied by another “magic” number - 0.00086. The correctness of this can be verified by taking the data from the previous example.
So, it was calculated that 0.05 Gcal = 58.15 kW/hour. Now it’s worth taking this result and multiplying it by 0.00086: 58.15 x 0.00086 = 0.050009.
Despite the slight difference, it almost completely coincides with the original data. As in previous calculations, it is necessary to take into account the fact that when working with particularly large volumes of substances, it will be necessary to convert not kilowatts, but megawatts into gigacalories. How is this done?
In this case, again you need to take into account that 1 mW = 1000 kW. Based on this, the decimal point in the “magic” number is moved by three zeros, and voila, it turns out to be 0.86. It is by this that you need to multiply to make the translation.
By the way, a small discrepancy in the answers is due to the fact that the coefficient 0.86 is a rounded version of the number 0.859845. Of course, for more accurate calculations it is worth using it. However, if we are talking only about the amount of energy used to heat an apartment or house, it is better to simplify.
What does the amount consist of?
Commercial relations between suppliers of various types of energy and residents are formally determined by Agreements.
According to these documents, when paying, the following are taken into account and paid:
- water flow measured by a water meter on a hot pipe;
- energy spent on heating it;
- consumer benefits;
- the amount stated in the receipt was obtained as the product of the tariff for hot water and its consumption according to the meter, taking into account benefits.
If the apartment is not connected to central heating, but is equipped with an autonomous heating system, the apartment owner pays additionally for gas or electricity, depending on the type of water heater.
What is hot water supply in a housing and communal services receipt?
DHW is a hot water supply intended for use by the population for domestic and industrial purposes. In addition to the abbreviation DHW, the payer may encounter the item “DHW ODN” in the receipt, where ODN stands for general household needs, which can be considered:
- maintaining optimal temperature in the entrance;
- resource used in preparation for the heating season;
- pressure testing (checking) of the pipeline;
- injection of water into the system for installation and repair work.
There are closed and open heating and hot water supply systems:
- If in an apartment building the water from the tap comes directly from the heating system, then it is an open system. In this case, water is much worse. Hot liquid and impurities gradually destroy communications. The end consumer receives a product of poor quality.
- Modern water supply is gradually switching to a closed system, when water for hot water supply is only heated by the coolant, without contacting it, i.e. Hot water is obtained by heating cold water using special equipment.
Procedure for detecting an error in a receipt
Errors in receipts for housing and communal services are not uncommon. There may be several reasons for this:
- failures and “lags” in the system;
- human factor;
- fraudulent actions of the management company.
If an error is found in the payment for heating and hot water supply, you should contact the company manager’s office. In a written statement they ask for a recalculation. Conscientious organizations, in order to avoid a scandal, go to meet the client, but among them there are not entirely honest ones. The responsible payer may be charged with the debt of a negligent neighbor who is in no hurry to pay his bills. Sometimes this is due to the desire for personal enrichment of the owners of the management company. In this case, you must immediately contact the prosecutor's office.
How to calculate the energy for heating water.
Initial data. The water is heated from 0.0°C (T1) to 100°C (T2). The difference is 100°.
Let's determine how much time it takes to heat 1000 liters of water with a heater power of 10 kW.
According to the formula:
| t=0.0017x | V(T2-T1) |
| W |
t= (0.00117 x V x (T2 - T1))/ W = (0.00117 x 1000 x (100 -0))/ 10 = 11.7 hours.
Where:
- t — time (in hours)
- V—tank volume (liters);
- T2 - temperature of heated water;
- T1—initial temperature of cold water;
- W is the electrical power of the heating element (kW).
Let us determine the power of the heating element required to heat 1000 liters of water from zero to 100°C per day.
Within 24 hours:
W= (0.00117 x V x (t2 - t1))/ T = (0.00117 x 1000 x (100 -0))/ 24 = 4.875 kilowatts per hour.
In one day you need to spend:
4.875 x 24 hours = 117 kilowatts of energy.
When heating one liter of water by one degree, 1.17 W of energy is consumed.
To heat 1 liter of water at 100°C you need:
117,000 W / 1000 l = 117 watts of energy
It turns out that to heat 1 liter of water by 1°C, you need to spend 1.17 W.
117W / 100°C = 1.17 W/liter
Let's remember this figure, it shows how much energy will be spent heating 1.0 liters of water in a kettle from zero degrees to 100°C. If the power of a conventional kettle is 1.0 kW (per hour), then the heating time will be approximately 7 minutes.
To simplify calculations, you can round up the amount of energy spent on heating 1.0 liters of water to 1.0 W in order to quickly make in your head all the necessary calculations on the energy costs for heating water, heating time, and the power required for this.
An example of a calculation without taking into account energy losses.
How long will it take to heat 1000 liters of water at a temperature from 10°C to 80°C with a 20 kW boiler?
Solution:
Heating requires energy consumption:
(T2 - T1) *1kW=(80-10)x1=70 kW
With a boiler power of 20 kW this will happen through:
70 kW/20=3.5 hours.
Let's give an example of calculating fuel consumption for heating a house.
When burning 50 cubic meters of natural gas, we get 500 kW
in 24 hours this is ~21 kW/hour.
This amount of energy can heat 100 m² of housing in winter for two cold days:
100m² * 0.1kW/m² * 24 hours = 240 kW per day
500kW / 240kW = ~2 days
As noted above, one cubic meter of methane gas, when burned, releases about 10 kW of energy (~10 kcal). According to building codes, consumption (on average in Siberia) is regulated at 1.0 kW per 10 m² of area (100 W/m²) with a room height of up to ~3 m and when it is -40°C outside. Accordingly, the estimated gas consumption when designing the heating of a house with an area of 100 m² in cold winter (if it is -40°C all the time) per month:
1 m³ x 24 hours x 30 days = 720 m³/month.
Since it is not -40°C all the time, therefore the approximate calculated consumption in practice will decrease by 2 times and will be approximately 360 m³, which at a gas price of about 6 rubles. will be 2160 rubles/month.
Heat losses, and therefore gas consumption, of course also depend on the insulation of the house.
It is also necessary to take into account gas costs for heating hot water and cooking.
The cost of energy in kW released when burning gas will be 6 rubles/10 kW = 0.6 rubles/kW. And the cost of electricity today is 3.0 rubles/kW. Therefore, it is 5 times cheaper to cook on a gas stove than on an electric one.
How to calculate it yourself: step-by-step instructions
To independently calculate the amount of the next payment for hot water supply, you need to use the formula:
Pi = ViP x Tcr, where:
- ViP is the water consumption for the payment period;
- Tkr is the value of the tariff for hot water supply;
- Pi — — payment for the control period.
Calculation example
The following indicators were used for the calculation:
- The amount of hot water used, measured by the water meter for February 2022, was 5 cubic meters (m3) (ViP - in the formula).
- The tariff for the region is 150 rubles. per 1 cubic meter (m3) (Tcr - in the formula).
The rent for the apartment is calculated as follows: 5 m3 x 150 rubles. = 750.00 rubles. The fee for hot water supply to an apartment is RUB 750.00.
Application of thermal energy
Let's look at a simple example of thermal energy. The heated element on the stove contains thermal energy, and the more you turn on the stove, the more internal energy it contains. At the most basic level, this thermal energy represents the movement of the molecules that make up the metal of the furnace element.
The faster the molecules move, the more internal thermal energy they contain.
Now let's place a pan of water on top of the heated element. What's going on? The stove is working. Here, "work" refers to "the movement of something when force is applied." Specifically, the heat energy from the oven causes the pot particles and ultimately the water to move faster. The internal energy of the heated element is transferred to the pan and, ultimately, to the water inside the pan. This transfer of thermal energy from the oven to the pan and into the water is called heat. It is very important to comply with these conditions. In this context, heat is the term we use to refer to the transfer of thermal energy from one object or system to another.
Thermal energy is the energy found within an object or within a system due to the movement of particles.
You may feel warm if you hold your hand over the stove. The heat, in turn, accelerates the molecules in the pot and water. If you place a thermometer in water as the water heats up, you can observe the temperature increase. Again, an increase in internal energy will result in an increase in temperature.