A car radiator is the most important part of the cooling system of a modern car. The performance of the engine as a whole depends on how it works. Of course, everyone knows the principle of operation of the internal combustion engine. It is based on a series of small explosions of fuel in the cylinder block. It is not surprising that as a result of this, the components and assemblies of the entire mechanism during operation are heated to very high temperatures and experience enormous loads. If the thermal energy generated during operation is not promptly removed from the engine compartment, then just one trip will be enough for certain components and assemblies of the internal combustion engine to completely fail. To prevent this from happening, the cooling system works.
Types and properties of heat-carrying liquids
The working fluid of any water system - the coolant - is a liquid that takes a certain amount of energy from the boiler and transfers it through pipes to heating devices - radiators or underfloor heating circuits. Conclusion: the efficiency of heating depends on the physical properties of the liquid mediator - heat capacity, density, fluidity, and so on.
In 95% of private houses, ordinary or treated water is used with a heat capacity of 4.18 kJ/kg•°C (in other units - 1.16 W/kg•°C, 1 kcal/kg•°C), which freezes at a temperature of about zero degrees. The advantages of traditional heating fluid are availability and low price, the main disadvantage is the increase in volume when frozen.
Crystallization of water is accompanied by expansion; cast iron radiators and metal-plastic pipelines are equally destroyed by ice pressure
The ice that forms in the cold literally splits pipes, boiler heat exchangers and radiators. To prevent the destruction of expensive equipment due to defrosting, 3 types of antifreeze made on the basis of polyhydric alcohols are poured into the system:
- Glycerin solution is the oldest type of non-freezing coolant. Pure glycerin is a transparent liquid of high viscosity, the density of the substance is 1261 kg/m³.
- An aqueous solution of ethylene glycol - dihydric alcohol with a density of 1113 kg/m³. The starting liquid is colorless and is inferior in viscosity to glycerin. The substance is toxic, the lethal dose of dissolved glycol when taken orally is about 100 ml.
- The same, based on propylene glycol - a transparent liquid with a density of 1036 kg/m³.
- Compositions based on the natural mineral - bischofite. We will analyze the characteristics and features of this chemical separately (below in the text).
Reference. Any non-freezing heating fluid contains a dye that gives the chemical a distinctive color. Poisonous ethylene glycol is usually colored red or yellow, propylene glycol is green (less often blue). Glycerin antifreeze is given a pink tint or left transparent. This classification is not mandatory and is not always observed.
Anti-freeze products are sold in two forms: ready-made solutions designed for a certain subzero temperature (usually -30 ° C), or concentrates, which the user dilutes with water himself. Let us list the properties of glycol antifreezes that affect the operation of heating networks:
- Low crystallization temperature. Depending on the concentration of polyhydric alcohol in an aqueous solution, the liquid begins to freeze at a temperature of minus 10...40 degrees. The concentrate crystallizes at 65 °C below zero.
- High kinematic viscosity. Example: for water this parameter is 0.01012 cm²/s, for propylene glycol - 0.054 cm²/s, the difference is 5 times.
- Increased fluidity and penetrating ability.
- The heat capacity of non-freezing solutions lies in the range of 0.8...0.9 kcal/kg °C (depending on concentration). On average, this parameter is 15% lower than that of water.
- Aggressiveness towards some metals, such as zinc.
- The substance foams when heated and quickly decomposes when boiling.
Propylene glycol antifreezes are usually painted green, and the prefix “ECO” is added to the marking.
In order for antifreezes to meet operational requirements, manufacturers add additive packages to glycol solutions - corrosion inhibitors and other elements that maintain the stability of the antifreeze and reduce foaming.
Additional functions
In addition to reducing the temperature of operating mechanisms, the cooler must meet some other requirements and perform parallel tasks.
- Protect the metal components of the engine from rusting. And this is important, because when metals interact with liquid compounds, corrosion is a very possible thing;
- Lubricate the pump bearing
. Otherwise, due to constant friction, it will gradually wear out and cease to perform its functions; - Increase the limit after which the liquid boils. It is very important in the summer heat, when engine temperatures are complemented by natural ones.
If you delve into this list, it will become clear why you cannot pour water into the radiator.
Two conditions for normal operation will be violated at once: metal parts will rust, and an unlubricated pump will fail. In a hopeless situation, just to get to the “place”, you can fill the radiator with water for a short time, but using it constantly means ruining the car.
About the pros and cons of glycol antifreeze
The main advantage of artificial coolants based on glycols is the preservation of the liquid phase at subzero temperatures. We list other positive aspects of using antifreeze in closed water heating systems:
- coolants do not contain calcium and magnesium salts, which form scale inside the heat exchangers;
- due to the penetrating ability of glycols, the effect of lubrication of moving parts occurs, ball valves and thermostatic valves do not sour, the fittings last longer;
- the boiling point of antifreeze 103-106 °C delays the moment of vaporization and airing in case of overheating of a solid fuel boiler;
- When the temperature drops below the freezing threshold, glycol solutions turn into a gel mass.
Note. The paragraph about scale implies that the “anti-freeze” is diluted with demineralized distilled water.
When glycol mixtures freeze, they form a slurry that is unable to rupture pipes and heat exchangers.
Let us explain the last 2 points. Ordinary water, often poured into the heating system of country houses, begins to boil at 96-98 ° C, actively releasing steam. If the circulation pump is on the TT boiler supply, the steam phase penetrates the chamber with the impeller, water pumping stops, and the boiler completely overheats. A higher boiling point of antifreeze will delay the moment of the accident.
Unlike water, glycol hardened in the cold does not expand and does not destroy pipe walls. In the event of freezing, the only unit that will suffer is the forced circulation pump. The crystallizing gel will jam the impeller and the motor will burn out.
Unfortunately, there are plenty of disadvantages to non-freezing substances:
- Ethylene glycol is poisonous and requires careful handling and disposal of the solution. Glycerin and polypropylene glycol are harmless.
- The heat capacity of the “anti-freeze” is 15% less. To deliver the required amount of heat to the batteries, the liquid flow will have to be increased.
- The viscosity of antifreeze creates additional hydraulic resistance. You will need a more powerful and expensive circulation pump.
- Good turnover is a double-edged sword. Glycols penetrate through the slightest leaks, from which plain water cannot flow.
- Coolants and additives decompose during operation, losing their frost-resistant properties and forming flakes of sediment. The maximum service life of 1 refill is 5 years, then the heating is flushed and replaced.
- When using antifreeze, many gas boiler manufacturers void the warranty of the purchased product.
Glycol liquids are poorly compatible with electric boilers. Instructions for the use of various antifreezes categorically do not recommend filling systems operating in conjunction with electrolysis heaters with antifreeze. That is, for electrode boilers of the “Galan” type, a special coolant developed by the specified company is needed.
Under rare circumstances, antifreeze can release flammable gas that breaks through the automatic air vent. Example: the heat source is an electric boiler, the heaters are Chinese-made aluminum radiators. Heating glycol causes a complex chemical reaction and gas formation. The fact is demonstrated in the video:
A little theory
If anyone has forgotten (or did not know), let us remind you: the engine’s operation is based on a series of explosions of a mixture of air and fuel. It is this that ensures the operation of the pistons and crankshaft. Naturally, the temperature during microexplosions rises to a very convincing figure of 2000°C. No material can survive such heat, so the engine must be continuously cooled.
There are 3 types of cooling:
- air. The most unreliable and ineffective. If anyone remembers, this happened on the Zaporozhets, which in the summer were constantly ventilated on the side of the road (with smoke) or drove with the hood tied with a string;
- liquid. The most common and most effective;
- combined, which combines both air and liquid cooling. A very promising and reliable development, but so far such systems are installed much less frequently than just liquid ones.
Whatever car you buy, there will certainly be either a second, or maybe a third type of cooling. This means a radiator where it needs to be filled.
{banner_content}
New mineral coolants
We decided to highlight the description of these liquids, since they are made based on the natural mineral - bischofite. The substance is a magnesium salt of hydrochloric acid, the full name is magnesium chloride hexahydrate. The manufacturer declares the following characteristics of the finished antifreeze, designed for a minimum temperature of minus 30 degrees:
- the color of the aqueous solution is light yellow, the density is 1117...1250 kg/m³;
- boiling threshold - 116 °C, freezing point - minus 30 °C;
- specific heat capacity - 0.77 kcal/kg •°С (3.23 kJ/kg•°С);
- thanks to the additives, there is no foaming and no aggressive effect on various seals - silicone, paronite, EPDM and BMS rubber;
- the substance is not toxic;
- In terms of viscosity and fluidity, the drug is very close to glycol chemicals.
Reference. The product appeared on the market after 2010. The price of liquid as of 2022 is about 1 USD. e. per liter of finished coolant (-30 ° C).
Compared to traditional glycol analogues, mineral antifreeze benefits due to its high boiling point, cost and health safety. The negative point is the increased density and low heat capacity, 23% worse than that of water.
The practical use of the coolant has revealed a number of shortcomings, as evidenced by reviews from homeowners:
- The fluidity of the solution is extremely high. There have been cases where antifreeze penetrated through the soldered joint of polypropylene pipes.
- Upon contact with air, the liquid fraction quickly evaporates, leaving a noticeable salt build-up. Similar phenomena are observed in heat exchangers and pipelines where air bubbles have penetrated.
- The substance reacts with bare metal on welds. Stalactites of iron and salt form inside the system, reducing the flow area and clogging the mud traps.
- In case of overheating, antifreeze turns into a liquid of unknown color, as shown in the photo.
User responses about this type of “anti-freeze” can be read on the well-known construction forum: https://www.stroimdom.com.ua/forum/showthread.php?t=157650
Taking into account the experience of users, we do not dare to recommend mineral antifreezes for use in heating systems of private houses. Perhaps over time, manufacturers will eliminate the above problems and the magnesium chloride solution will be able to compete on equal terms with glycols.
Gunk Radiator Sealer Super
Price: 627 rub.
Not cheap, but quite effective in the form of a thick brown suspension. “Super composition” from the American manufacturer Radiator Specialty Company reliably closes holes of various sizes in the radiator and is quite suitable for urgent repairs.
This sealant is suitable for both cars and trucks. The drug is able to restore aluminum, brass, and radiators made from composite materials. However, the product in a 443 ml canister also has its own “fly in the ointment” in the form of the toxic substance sodium borate decahydrate.
Advantages:
- performance;
- compatible with all types of antifreeze.
Flaws:
- leaves behind a lot of sediment, which can harm worn-out or thickly deposited areas where antifreeze passes;
- Do not allow the drug to come into contact with the skin, eyes or mouth; high price.
Choosing an “anti-freeze” for heating
Tip number one: buy and fill in antifreeze only in extreme cases - for periodic heating of remote country houses, garages or buildings under construction. Try to use water - regular and distilled, this is the least troublesome option.
When choosing a frost-resistant coolant, follow the following recommendations:
- If your budget is limited, take ethylene glycol from any well-known brand - “Teply Dom”, Dixis, Spektrogen Teplo Coolant, Bautherm, Termo Tactic or “Thermagent”. The cost of the concentrate -65 °C from Dixis is only 1.3 USD. e. (90 rubles) per 1 kg.
- If there is a danger of antifreeze getting into household water (for example, through an indirect heating boiler, double-circuit boiler), or you are very concerned about the environment and safety, buy harmless propylene glycol. But keep in mind: the price of the chemical is higher; a ready-made Dixis solution (minus 30 degrees) will cost 100 rubles (1.45 USD) per kilogram.
- For large heating systems, we recommend using premium class HNT coolant. The liquid is made on the basis of propylene glycol, but it has an increased service life of 15 years.
- Do not buy glycerin solutions at all. Reasons: sedimentation in the system, too high viscosity, tendency to foam, a large number of low-quality products made from technical glycerin.
In the light of the flashlight, tiny white flakes are visible - a sediment of technical glycerin - Electrode boilers require a special liquid, for example, HNT-35. Before use, be sure to consult with a representative of the manufacturer.
- Do not confuse automobile antifreezes with chemicals used in heating systems. Yes, both formulations are glycol-based, but the additive packages are completely different. Engine coolant is not compatible with residential water heating.
- For open and gravity-flow heating systems, it is better to use water, or, in extreme cases, propylene glycol diluted at minus 20 °C.
- If the heating distribution is made with galvanized pipes, there is no point in purchasing glycol mixtures. The substance will deal with zinc, lose the package of additives and quickly degrade.
Clarification. It is not profitable to use frost-resistant liquid for an open heating system. Hot antifreeze will evaporate into the atmosphere through the expansion tank, the antifreeze will have to be constantly topped up, and money will be spent. It is unacceptable to pump in ethylene glycol, since its vapors are toxic.
There is a lot of debate about the harmfulness of ethylene glycol compounds, including on the pages of construction forums. Without denying the harmful effects of the chemical on human health, let us draw attention to a convincing fact.
Homeowners whose closed systems are installed well have been using inexpensive glycol for years without any problems. Let's listen to the expert's opinion in the video:
What to put in the car?
The best thing is what is recommended in the service literature for your car. But since the information in the book may be outdated, and technology has moved forward, you can refer to the following recommendations:
- Green antifreeze G11 can be poured if you have a system with aluminum components (radiators, pipes). Aluminum is more susceptible to oxidation and cavitation, so it needs a reliable protective layer of additives on the walls. It needs to be changed every 2 years.
- Red G12, pink G12+, G12++ can be poured both into systems with copper/brass components and into systems where aluminum predominates, but G11 and G12++ are more suitable for aluminum
- Suitable for absolutely all G13 systems, which are usually painted yellow. It is environmentally friendly and durable over time. Replacement according to the manufacturer within 5 years of operation.
- It is not recommended to pour antifreeze into modern cars, since not only silicates are used as additives, but also phosphates and borates, which are not suitable for modern cooling systems.
Instructions for use
If your system previously ran on water, switching to antifreeze will not be easy. Theoretically, radiators with a boiler can be emptied and filled with cold-resistant coolant, but in practice the following will happen:
- due to lower heat capacity, the output of batteries and the efficiency of heating rooms will decrease;
- due to viscosity, the load on the pump will increase, coolant flow will drop, and less heat will reach the radiators;
- antifreeze expands more than water, so the capacity of the old tank will not be enough, the pressure in the network will rise;
- To improve the situation, you will have to increase the temperature on the boiler, which will lead to excessive fuel consumption and increased pressure.
Addition. After filling the liquid, the old connections sealed with flax and paint are guaranteed to flow.
Leaking joints must be repacked, sealing the threads with dry flax or thread with sealant
. In order for heating to function normally using a chemical coolant, you need to calculate in advance or remake the existing system according to the new requirements:
- Select the capacity of the expansion tank at the rate of 15% of the total volume of liquid (water was 10%);
- The pump performance is assumed to be 10% more, and the generated pressure is assumed to be 50% more. Let us explain with an example: if previously there was a unit with a working pressure of 0.4 Bar (4 meters of water column), then use a 0.6 Bar pump for antifreeze.
- In order to operate the boiler in optimal mode and not raise the temperature of the coolant, it is advisable to add 1-3 (depending on power) sections to each battery.
- Pack all joints with dry flax or use high-quality pastes - sealants such as LOCTITE, ABRO or Hermesil.
- When purchasing shut-off and control valves, consult with the seller about the resistance of rubber seals to glycol mixtures.
- Pressure test the system again by filling the pipes and heating equipment with water.
- When starting the boiler unit at negative temperatures, set the minimum power. Cold antifreeze needs to be warmed up slowly.
Advice. The total amount of coolant is easy to calculate - the cross-sectional area of the pipe is multiplied by its length, the capacity of the boiler and radiators is indicated in the product data sheets. Find out how to properly place and connect the expansion tank in our separate publication.
Before pumping in frost-resistant liquid, fill in water and test the pipelines with a pressure exceeding the operating pressure by 25%. The
concentrated coolant must be diluted with water, ideally with distillate. Do not rely on an excessive reserve of frost resistance - the more water you add, the better the heating will work. Recommendations for preparing coolant:
- For heating elements, electric and gas double-circuit heat generators, prepare the mixture at minus 20 degrees. A more concentrated solution may foam upon contact with the heater, and carbon deposits will form on the surface of the heating element.
- In other cases, mix the components at freezing point according to the table below. Proportions are indicated per 100 liters of coolant.
- If there is no distillate, first conduct an experiment - dilute the concentrate in a jar with plain water. If you see a precipitate of white flakes - a product of the decomposition of inhibitors and additives, this water should not be used.
- A similar check is done before mixing antifreeze from two different manufacturers. It is unacceptable to dilute ethylene glycol with propylene composition.
- Prepare the coolant immediately before pouring.
The ratio of concentrate and water is given per 100 liters.
To find out the amount of ingredients for a volume of 150 liters, multiply the given figures by a factor of 1.5. The maximum service life of any non-freezing substance in pipes and heating radiators is 5 years. At the end of the specified period, the liquid is drained, the system is flushed twice and filled with fresh antifreeze.
Design features
To a person unfamiliar with the design of even the most primitive internal combustion engine, the cooling system may seem like an extremely complex device. However, in reality, everything is not so scary and to understand how it works, it is enough to study only the design features of the device. It consists of the following parts:
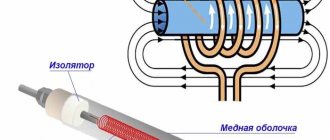
- Radiator. Its structure is similar to the most common battery in a city apartment. The only difference is that the metal tubes that make up car radiators are much thinner and made of pure copper, which can become a reservoir even for aggressive chemical liquids.
- The fan is installed immediately behind the radiator grille. It turns on at the moment when the radiator can no longer cope with cooling the liquid and creates an additional cooling effect using air flows from the street.
- Wire channels and tubes in the engine - provide fluid access to all engine elements that require cooling.
- Pumps and pumps responsible for the movement of fluid through channels.
- Expansion tank - comes into operation when the liquid gets very hot and, according to the laws of physics, begins to expand. In fact, the tank absorbs excess coolant and protects the system from leakage. By the way, it is in it that the coolant is initially poured, which will circulate through the system.
It should be added that in any cooling system there is a small and large circle of liquid circulation. Initially, when the engine is just started, circulation occurs in a small circle. This happens until the motor reaches the desired temperature, at which point special bypass valves open and the liquid flows into the large circle. It is on this that the heating of the interior occurs, so the heat in the car always becomes only after the engine has completely warmed up.
And it is also very important for the system to work by choosing the right fluid. Let's talk about how to do this.
Finally, about coolants for electrode boilers
Electric water heaters of this type operate on the principle of a “soldier’s boiler” consisting of two blades connected to a 220 volt network. Water simultaneously serves as a coolant and electrolyte; heating occurs due to its conductivity, which depends on the content of magnesium and calcium salts.
This is why electrode boilers do not work with distillate and significantly lose power with under-salted water. According to the passport of the Galan heater, the resistance of the working fluid should be no more than 3200 Ohms per 1 cm.
If you pour regular ethylene glycol into an electrolysis heat generator, the substance will enter into a chemical reaction, foam and lose additives against corrosion and scale formation. The problem can be solved in 2 ways:
- A special antifreeze designed for electrode-type units is purchased. Special additives that resist foaming are dissolved in the working environment.
- A saline solution of the required concentration is prepared, as shown in the video below. Such water will begin to crystallize at a lower temperature, although it cannot be compared with antifreeze in terms of frost resistance.
You should pay attention to the preparation of tap water - pass it through a filter and let it sit for 1-3 days. A good solution is to buy a corrosion inhibitor separately and add it to the coolant for the heating system in advance.
Flushing the system
The cooling system should not be flushed frequently - once a year or when signs appear indicating the need to replace the antifreeze.
If it is necessary to add coolant in a volume of about 30% of the technological minimum, then it should be completely replaced.
If the check shows that the antifreeze has turned black or become thicker, it is changed. Replacement is also carried out when:
- regular engine overheating;
- the end of the service life of the poured composition;
- supply of cold air from the stove;
- early operation of the fan and its operation at high speeds;
- unsatisfactory functioning of the pump;
- when the safety release valve on the expansion tank is activated.
Acid-containing compounds are used to remove rust, and alkaline compounds are used to combat alkaline deposits. Work should be carried out correctly in warm weather. Draining is carried out through a special hole. Residues are removed through an additional outlet, which is located on the bottom and is often covered with a protective panel.
What you can fill in to flush the system:
- water;
- citric acid;
- acetic acid;
- lactic acid;
- caustic soda;
- special equipment (LAVR, LIQUI MOLY Kuhler-Reiniger, Hi-Gear Radiator Flush-7 minute).
Acids are usually pre-diluted with water. The technology of work depends on the type of product chosen.
Liqui Moly
Declared manufacturer: Germany
Approximate price: 200 rub.
Metal-containing drug. The sediment after draining shone with sparkles of all colors. A clear leader in the speed of sealing holes, and the leaks, having stopped, never resumed. The level of residual deposits is average.
+ Best operating efficiency.
- Price.
1 place. Radiator sealant “BBF Super”
"BBF Super"
Declared manufacturer: Russia
Approximate price: 55 rub.
The white emulsion works classically - it consistently and relatively quickly closes the holes from smaller to larger. There are very few residual deposits. After opening, neat fungi of polymer plugs are visible in the place of the holes. And at the same time - the cheapest composition among those tested!
+ The best price/quality combination. Lowest sediment level.
- Not detected.
Final table (click to open full size):