The tables present the mass specific heat of combustion of fuel (liquid, solid and gaseous) and some other combustible materials. The following fuels were considered: coal, firewood, coke, peat, kerosene, oil, alcohol, gasoline, natural gas, etc.
List of tables:
- Specific heat of combustion of solid fuel
- Specific heat of combustion of liquid fuel
- Specific heat of combustion of gaseous fuel
- Specific heat of combustion of some combustible materials
During the exothermic reaction of fuel oxidation, its chemical energy is converted into thermal energy with the release of a certain amount of heat. The resulting thermal energy is usually called the heat of combustion of the fuel. It depends on its chemical composition, humidity and is the main indicator of fuel. The heat of combustion of fuel per 1 kg of mass or 1 m3 of volume forms the mass or volumetric specific heat of combustion.
The specific heat of combustion of a fuel is the amount of heat released during the complete combustion of a unit mass or volume of solid, liquid or gaseous fuel. In the International System of Units, this value is measured in J/kg or J/m3.
The specific heat of combustion of a fuel can be determined experimentally or calculated analytically. Experimental methods for determining calorific value are based on practical measurement of the amount of heat released when a fuel burns, for example in a calorimeter with a thermostat and a combustion bomb. For fuel with a known chemical composition, the specific heat of combustion can be determined using the periodic formula.
There are higher and lower specific heats of combustion. The higher calorific value is equal to the maximum amount of heat released during complete combustion of the fuel, taking into account the heat expended on the evaporation of moisture contained in the fuel. The lowest heat of combustion is less than the highest value by the amount of heat of condensation of water vapor, which is formed from the moisture of the fuel and hydrogen of the organic mass, which turns into water during combustion.
To determine fuel quality indicators, as well as in thermal calculations, the lowest specific calorific value is usually used , which is the most important thermal and operational characteristic of the fuel and is shown in the tables below.
Specific heat of combustion of solid fuels (coal, firewood, peat, coke)
The table presents the values of the specific heat of combustion of dry solid fuel in the dimension MJ/kg. Fuel in the table is arranged by name in alphabetical order.
Of the solid fuels considered, coking coal has the highest calorific value - its specific heat of combustion is 36.3 MJ/kg (or in SI units 36.3·106 J/kg). In addition, high calorific value is characteristic of hard coal, anthracite, charcoal and brown coal.
Fuels with low energy efficiency include wood, firewood, gunpowder, milling peat, and oil shale.
For example, the specific heat of combustion of firewood is 8.4...12.5, and that of gunpowder is only 3.8 MJ/kg. Specific heat of combustion of solid fuels (coal, firewood, peat, coke)
| Fuel | Specific heat of combustion, MJ/kg |
| Anthracite | 26,8…34,8 |
| Wood pellets (pellets) | 18,5 |
| Dry firewood | 8,4…11 |
| Dry birch firewood | 12,5 |
| Gas coke | 26,9 |
| Blast coke | 30,4 |
| Semi-coke | 27,3 |
| Powder | 3,8 |
| Slate | 4,6…9 |
| Oil shale | 5,9…15 |
| Solid rocket fuel | 4,2…10,5 |
| Peat | 16,3 |
| Fibrous peat | 21,8 |
| Milled peat | 8,1…10,5 |
| Peat crumb | 10,8 |
| Brown coal | 13…25 |
| Brown coal (briquettes) | 20,2 |
| Brown coal (dust) | 25 |
| Donetsk coal | 19,7…24 |
| Charcoal | 31,5…34,4 |
| Coal | 27 |
| Coking coal | 36,3 |
| Kuznetsk coal | 22,8…25,1 |
| Chelyabinsk coal | 12,8 |
| Ekibastuz coal | 16,7 |
| Frestorf | 8,1 |
| Slag | 27,5 |
Firewood
These are sawn or chopped pieces of wood, which, when burned in furnaces, boilers and other devices, generate thermal energy.
For ease of loading into the firebox, wood material is cut into individual elements up to 30 cm long. To increase the efficiency of their use, the firewood must be as dry as possible and the combustion process must be relatively slow. In many respects, wood from hardwoods such as oak and birch, hazel and ash, and hawthorn are suitable for heating premises. Due to the high resin content, increased burning rate and low calorific value, coniferous trees are significantly inferior in this regard.
Specific heat of combustion of liquid fuels (alcohol, gasoline, kerosene, oil)
A table is given of the specific heat of combustion of liquid fuel and some other organic liquids. It should be noted that fuels such as gasoline, aviation kerosene, diesel fuel and oil have a high heat release during combustion.
The specific heat of combustion of alcohol and acetone is significantly lower than traditional motor fuels.
In addition, liquid rocket fuel and ethylene glycol have a relatively low calorific value - with complete combustion of 1 kg of these hydrocarbons, an amount of heat will be released equal to 9.2 and 13.3 MJ, respectively. Specific heat of combustion of liquid fuels (alcohol, gasoline, kerosene, oil)
| Fuel | Specific heat of combustion, MJ/kg |
| Acetone | 31,4 |
| Gasoline A-72 (GOST 2084-67) | 44,2 |
| Aviation gasoline B-70 (GOST 1012-72) | 44,1 |
| Gasoline AI-93 (GOST 2084-67) | 43,6 |
| Benzene | 40,6 |
| Winter diesel fuel (GOST 305-73) | 43,6 |
| Summer diesel fuel (GOST 305-73) | 43,4 |
| Liquid rocket fuel (kerosene + liquid oxygen) | 9,2 |
| Aviation kerosene | 42,9 |
| Kerosene for lighting (GOST 4753-68) | 43,7 |
| Xylene | 43,2 |
| High sulfur fuel oil | 39 |
| Low sulfur fuel oil | 40,5 |
| Low-sulfur fuel oil | 41,7 |
| Sulphurous fuel oil | 39,6 |
| Methyl alcohol (methanol) | 21,1 |
| n-Butyl alcohol | 36,8 |
| Oil | 43,5…46 |
| Methane oil | 21,5 |
| Toluene | 40,9 |
| White spirit (GOST 313452) | 44 |
| Ethylene glycol | 13,3 |
| Ethyl alcohol (ethanol) | 30,6 |
Comparison of energy intensity of different types of fuel
When comparing the energy value of the main types of solid, liquid and gaseous fuels, it can be established that one liter of gasoline or diesel fuel corresponds to 1.3 m³ of natural gas, one kilogram of coal - 0.8 m³ of gas, one kg of firewood - 0.4 m³ of gas.
The heat of combustion of a fuel is the most important indicator of efficiency, but the breadth of its distribution in areas of human activity depends on the technical capabilities and economic indicators of use.
Specific heat of combustion of gaseous fuels and combustible gases
A table is presented of the specific heat of combustion of gaseous fuel and some other combustible gases in the dimension MJ/kg.
Of the gases considered, hydrogen has the highest mass specific heat of combustion. The complete combustion of one kilogram of this gas will release 119.83 MJ of heat. Also, fuel such as natural gas has a high calorific value - the specific heat of combustion of natural gas is 41...49 MJ/kg (for pure methane it is 50 MJ/kg). Specific heat of combustion of gaseous fuel and combustible gases (hydrogen, natural gas, methane)
| Fuel | Specific heat of combustion, MJ/kg |
| 1-Butene | 45,3 |
| Ammonia | 18,6 |
| Acetylene | 48,3 |
| Hydrogen | 119,83 |
| Hydrogen, mixture with methane (50% H2 and 50% CH4 by mass) | 85 |
| Hydrogen, mixture with methane and carbon monoxide (33-33-33% by weight) | 60 |
| Hydrogen, mixture with carbon monoxide (50% H2 50% CO2 by mass) | 65 |
| Blast furnace gas | 3 |
| Coke Oven Gas | 38,5 |
| Liquefied hydrocarbon gas LPG (propane-butane) | 43,8 |
| Isobutane | 45,6 |
| Methane | 50 |
| n-Butane | 45,7 |
| n-Hexane | 45,1 |
| n-Pentane | 45,4 |
| Associated gas | 40,6…43 |
| Natural gas | 41…49 |
| Propadiene | 46,3 |
| Propane | 46,3 |
| Propylene | 45,8 |
| Propylene, mixture with hydrogen and carbon monoxide (90%-9%-1% by weight) | 52 |
| Ethane | 47,5 |
| Ethylene | 47,2 |
Burning rules
When a consumer becomes familiar with the combustion temperature of a particular coal, he needs to take into account that manufacturers indicate only those figures that are relevant for ideal conditions. Of course, it is simply impossible to recreate the necessary parameters in an ordinary household boiler or oven. Modern heat generators made of metal or brick are simply not designed for such high temperatures, since the main coolant in the system can quickly boil. That is why the combustion parameters of a particular fuel are determined by its combustion mode.
In other words, it all depends on the intensity of the air supply. Both fossil and charcoal heat a room well if the oxygen supply level reaches 100%. To restrict the air flow, you can use a special damper/gate. This approach makes it possible to create the most favorable combustion conditions for filled fuel (up to 950˚C).
If coal is used in a solid fuel boiler, then boiling of the coolant must not be allowed. The main danger is that the safety valve may simply not work, which can lead to a large explosion. In addition, a mixture of water and hot steam has a bad effect on the functionality of the circulation pump. Experts have developed two most effective methods that allow you to control the combustion process:
- Crushed or powdered fuel must enter the boiler exclusively in a dosed volume (the same scheme applies as in pellet devices).
- The main energy carrier is loaded into the firebox, after which the intensity of the air supply is adjusted.
Specific heat of combustion of some combustible materials
A table is provided of the specific heat of combustion of some combustible materials (building materials, wood, paper, plastic, straw, rubber, etc.).
Materials with high heat release during combustion should be noted. Such materials include: rubber of various types, expanded polystyrene (foam), polypropylene and polyethylene. Specific heat of combustion of some combustible materials
| Fuel | Specific heat of combustion, MJ/kg |
| Paper | 17,6 |
| Leatherette | 21,5 |
| Wood (bars with 14% moisture content) | 13,8 |
| Wood in stacks | 16,6 |
| Oak wood | 19,9 |
| Spruce wood | 20,3 |
| Wood green | 6,3 |
| Pine wood | 20,9 |
| Capron | 31,1 |
| Carbolite products | 26,9 |
| Cardboard | 16,5 |
| Styrene butadiene rubber SKS-30AR | 43,9 |
| Natural rubber | 44,8 |
| Synthetic rubber | 40,2 |
| Rubber SKS | 43,9 |
| Chloroprene rubber | 28 |
| Polyvinyl chloride linoleum | 14,3 |
| Double-layer polyvinyl chloride linoleum | 17,9 |
| Polyvinyl chloride linoleum on a felt basis | 16,6 |
| Warm-based polyvinyl chloride linoleum | 17,6 |
| Fabric-based polyvinyl chloride linoleum | 20,3 |
| Rubber linoleum (Relin) | 27,2 |
| Paraffin paraffin | 11,2 |
| Polystyrene foam PVC-1 | 19,5 |
| Foam plastic FS-7 | 24,4 |
| Foam plastic FF | 31,4 |
| Expanded polystyrene PSB-S | 41,6 |
| Polyurethane foam | 24,3 |
| Fiberboard | 20,9 |
| Polyvinyl chloride (PVC) | 20,7 |
| Polycarbonate | 31 |
| Polypropylene | 45,7 |
| Polystyrene | 39 |
| High pressure polyethylene | 47 |
| Low-pressure polyethylene | 46,7 |
| Rubber | 33,5 |
| Ruberoid | 29,5 |
| Channel soot | 28,3 |
| Hay | 16,7 |
| Straw | 17 |
| Organic glass (plexiglass) | 27,7 |
| Textolite | 20,9 |
| Tol | 16 |
| TNT | 15 |
| Cotton | 17,5 |
| Cellulose | 16,4 |
| Wool and wool fibers | 23,1 |
Sources:
- Abryutin A. A. et al. Thermal calculation of boilers. Normative method.
- GOST 147-2013 Solid mineral fuel. Determination of the higher calorific value and calculation of the lower calorific value.
- GOST 21261-91 Petroleum products. Method for determining the higher calorific value and calculating the lower calorific value.
- GOST 22667-82 Natural flammable gases. Calculation method for determining the calorific value, relative density and Wobbe number.
- GOST 31369-2008 Natural gas. Calculation of calorific value, density, relative density and Wobbe number based on component composition.
- Zemsky G. T. Flammable properties of inorganic and organic materials: reference book M.: VNIIPO, 2016 - 970 p.
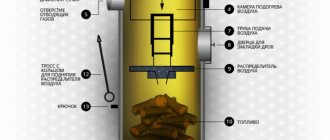
Charcoal how to use
You need to light charcoal without using any chemicals: the unpleasant smell from it will remain as long as the fuel burns. Therefore, we take a piece of paper, crumple it, build a few thin dry splinters around the paper in a “hut”, set the paper on fire, wait for the splinters to burn, put some dry firewood on top. When they burn well, you can add charcoal. Moreover, it needs to be folded in a slide. This way it flares up better. If we are talking about barbecue and cooking, then so that the charcoal flares up evenly, we put those pieces that are on the edges of the hill on top. Those that were closer to the center end up on the edge. So we wait until all the pieces are covered with a white coating and flames stop appearing above them. Now you can barbecue.
How to light charcoal? Using paper and thin wood chips or...a hair dryer
There is a way to light charcoal without paper, matches or wood. All you need is a hair dryer and an electrical outlet. All. No paper, no matches. Take a hairdryer, turn it on to maximum, direct the air flow onto the piled charcoal. The first ember will flare up in about half a minute, then the rest will take over, and in about five minutes everything will be burning.