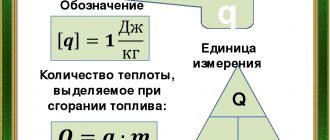
When a certain amount of fuel is burned, a measurable amount of heat is released. According to the International System of Units, the value is expressed in Joules per kg or m3. But the parameters can also be calculated in kcal or kW. If the value is related to a unit of fuel measurement, it is called specific.
What affects the calorific value of various fuels? What is the value of the indicator for liquid, solid and gaseous substances? The answers to the above questions are described in detail in the article. In addition, we have prepared a table displaying the specific heat of combustion of materials - this information will be useful when choosing a high-energy type of fuel.
General information about calorific value
The release of energy during combustion should be characterized by two parameters: high efficiency and the absence of production of harmful substances.
Artificial fuel is obtained by processing natural - biological fuel. Regardless of the state of aggregation, substances in their chemical composition have a flammable and non-flammable part. The first is carbon and hydrogen. The second consists of water, mineral salts, nitrogen, oxygen, and metals.
Based on their state of aggregation, fuel is divided into liquid, solid and gas. Each group further branches into a natural and artificial subgroup (+)
When 1 kg of such a “mixture” is burned, different amounts of energy are released. Exactly how much of this energy will be released depends on the proportions of these elements - the combustible part, humidity, ash content and other components.
The heat of combustion of fuel (HCT) is formed from two levels - the highest and the lowest. The first indicator is obtained due to water condensation; in the second, this factor is not taken into account.
The lowest TCT is needed to calculate the need for fuel and its cost; with the help of such indicators, heat balances are compiled and the efficiency of fuel-burning installations is determined.
TST can be calculated analytically or experimentally. If the chemical composition of the fuel is known, the periodic formula is applied. Experimental techniques are based on the actual measurement of heat from fuel combustion.
In these cases, a special combustion bomb is used - a calorimetric one together with a calorimeter and a thermostat.
Features of the calculations are individual for each type of fuel. Example: TCT in internal combustion engines is calculated from the lowest value, because the liquid does not condense in the cylinders.
TST is installed using a bomb calorimeter. Compressed oxygen is saturated with water vapor. A sample of fuel is placed in such an environment and the results are determined.
Each type of substance has its own TST due to the characteristics of its chemical composition. The values vary significantly, the range of fluctuations is 1,000–10,000 kCal/kg.
When comparing different types of materials, the concept of reference fuel is used; it is characterized by a lower TCT of 29 MJ/kg.
The procedure for calculating the pellet consumption required for heating
</>
Pellets intended for use in solid fuel boilers are sold in prepackaged bags, each of which indicates the weight of the fuel inside. Therefore, calculating the consumption of pellets required to generate 1 kW of thermal energy or heat 1 m² of space will not be difficult.
A simplified calculation of pellet consumption looks like this. High-quality pellets release a significant amount of heat when burned; 1 kg of pellets generates about 5 kW of thermal energy. This means that to obtain 1 kW of heat, you will need to burn 200 grams of fuel. As you know, to heat 1 m² of area, 100 W of thermal energy is required. By performing simple arithmetic operations, it can be calculated that when burning 20 grams of fuel, the required 100 W of thermal energy will be obtained. It should be noted that this calculation is valid for rooms whose ceiling heights range from 2.8 to 3 meters. A more accurate calculation differs from a simplified one. The fact is that the obtained value of pellet consumption for heating 1 m² of room would be fair if the boiler had an efficiency coefficient of 1 or 100%. Unfortunately or fortunately, there are no devices in nature that have 100% efficiency; a pellet boiler is no exception. Therefore, when calculating pellet consumption, it is necessary to take into account the efficiency of the boiler, the numerical value of which is 85% or 0.85. Consequently, when 1 kg of granules is burned in a furnace, not 5 kW of thermal energy will be released, but 5000 W x 0.85 = 4250 W or 4.25 kW. Carrying out further calculations, we find that to generate 1 kW of heat, 1000/4.25 = 135 grams of fuel will be required. The second drawback of the simplified calculation is that the ratio regulating the cost of 100 W of thermal energy per 1 m² is valid only for the situation when the room has the lowest temperature for 5 days. In reality, during the heating season, only 50 W of heat is needed to heat 1 m² of any room in a private house. If you calculate the consumption of pellets required to heat 1 m² of area for 1 hour, you will get a very small and inconvenient figure for further calculations. Therefore, it is better to calculate the amount of fuel that allows you to heat 1 m² for one day. So, to heat 1 m² of area throughout the day, it is necessary for the boiler to generate 50 W x 24 hours = 1200 W or 1.2 kW. In order to release the specified amount of thermal energy, the boiler must burn 1200 W / 4.25 kW/kg = 0.28 kg or 280 g.
Having calculated the specific fuel consumption, it is not difficult to calculate the values necessary for financial calculations. The owner of a house with an area of 100 m² will spend:
- for one day – 0.28 x 100 = 28 kg of granules;
- for one month – 28 x 30 = 840 kg.
It should be noted that the obtained value may change in one direction or another, since the amount of fuel consumed is directly dependent on the quality of insulation of the walls of the house. According to reviews from owners, monthly consumption of pellets in a well-insulated house of 100 m² does not exceed 550 kg. So, knowing the specific consumption of pellets for a solid fuel boiler that uses pellets to generate heat, it is not difficult to calculate the required amount of fuel for houses of various sizes:
- 100 m² - with poor insulation, the consumption will be 840 kg, with high-quality insulation - 550 kg;
- 150 m² - with insufficient insulation - 1260 kg, with good insulation - 825 kg;
- 200 m² - 1680 kg and 1100 kg, respectively.
Also, private homeowners should pay attention to the fact that currently many models of pellet boilers are equipped with special controllers - electronic devices that automatically calculate fuel consumption during a given period. The calculated information is provided to the user in a convenient form on the device display.
Calorific value of solid materials
This category includes wood, peat, coke, oil shale, briquette and pulverized fuel. The main component of solid fuel is carbon.
Features of different types of wood
Maximum efficiency from the use of firewood is achieved provided that two conditions are met - dry wood and a slow combustion process.
Pieces of wood are sawn or chopped into pieces up to 25-30 cm long so that firewood can be conveniently loaded into the firebox
Oak, birch, and ash bars are considered ideal for wood-burning stove heating. Hawthorn and hazel are characterized by good indicators. But coniferous trees have a low calorific value, but a high burning rate.
How different rocks burn:
- Beech, birch, ash, and hazel are difficult to melt, but they can burn wet due to their low moisture content.
- Alder and aspen do not form soot and “know how” to remove it from the chimney.
- Birch requires a sufficient amount of air in the firebox, otherwise it will smoke and deposit resin on the walls of the pipe.
- Pine contains more resin than spruce, so it sparks and burns hotter.
- Pear and apple trees split easier than others and burn well.
- The cedar gradually turns into smoldering coal.
- Cherry and elm smoke, and sycamore is difficult to split.
- Linden and poplar burn out quickly.
TST indicators of different breeds strongly depend on the density of specific breeds. 1 cubic meter of firewood is equivalent to approximately 200 liters of liquid fuel and 200 m3 of natural gas. Wood and firewood fall into the low energy efficiency category.
Effect of age on coal properties
Coal is a natural material of plant origin. It is mined from sedimentary rocks. This fuel contains carbon and other chemical elements.
In addition to the type, the heat of combustion of coal is also influenced by the age of the material. Brown belongs to the young category, followed by stone, and anthracite is considered the oldest.
The moisture content is also determined by the age of the fuel: the younger the coal, the higher its moisture content. Which also affects the properties of this type of fuel
The process of coal combustion is accompanied by the release of substances that pollute the environment, and the boiler grates are covered with slag. Another unfavorable factor for the atmosphere is the presence of sulfur in the fuel. This element, upon contact with air, transforms into sulfuric acid.
Manufacturers manage to reduce the sulfur content in coal as much as possible. As a result, TST differs even within the same species. The production geography also influences the performance. Not only pure coal, but also briquetted slag can be used as solid fuel.
The highest fuel capacity is observed in coking coal. Stone, charcoal, brown coal, and anthracite also have good characteristics.
Characteristics of pellets and briquettes
This solid fuel is produced industrially from various wood and plant waste.
Shredded shavings, bark, cardboard, and straw are dried and turned into granules using special equipment. In order for the mass to acquire a certain degree of viscosity, a polymer, lignin, is added to it.
Pellets have an acceptable cost, which is influenced by high demand and features of the manufacturing process. This material can only be used in boilers designed for this type of fuel.
Briquettes differ only in shape; they can be loaded into furnaces and boilers. Both types of fuel are divided into types based on raw materials: round timber, peat, sunflower, straw.
Pellets and briquettes have significant advantages over other types of fuel:
- complete environmental friendliness;
- possibility of storage in almost any conditions;
- resistance to mechanical stress and fungus;
- uniform and long burning;
- optimal granule size for loading into a heating device.
Eco-friendly fuel is a good alternative to traditional heat sources, which are not renewable and have an adverse effect on the environment. But pellets and briquettes are characterized by an increased fire hazard, which should be taken into account when organizing a storage location.
If you wish, you can set up the production of fuel briquettes yourself; more details can be found in this article.
Features of pellet heating systems
In order to evaluate the effectiveness of pellet heating, you need to know about the difference that exists between ordinary firewood and pellets. During the production of pellets, wood processing waste is used. The raw materials for the production of pellets or ordinary sawdust are first thoroughly dried, then treated with steam, resulting in the formation of a viscous mass, from which cylindrical granules with a length of about 70 mm and a diameter of 6 to 8 mm are formed under a pressure of 300 atmospheres. Boilers supplied to the market by manufacturers of pellet equipment differ from their counterparts that use ordinary firewood as fuel by a higher degree of heat transfer. Quantitative indicators of the calorific value of various types of solid fuel are shown in the table.
| Type of fuel | Heat of combustion (kW/kg) |
| Firewood | 2,84 |
| Fuel briquettes | 4,7 |
| Pellets | 4,99 |
In addition to high heat transfer, pellet boilers have another advantage - their combustion chamber is loaded automatically. Automatic fuel supply is implemented as follows:
- A certain supply of pellets is stored in a special hopper made of stainless steel. The presence of a large-volume bunker allows you to refuel it once every few days.
- The fuel enters the boiler through a flexible cable and an auger located inside it. The granules' own weight causes the auger to rotate, which ensures their supply to the distribution chamber of the boiler in the required quantity.
- Next, from the distribution chamber, the pellets enter the air burner zone, where they are incompletely burned, accompanied by the release of wood gas.
- The main source of high heat transfer is wood gas, which burns completely in the afterburner.
This design of the boiler allows its owner, during continuous operation of the equipment, to fill the bunker with fuel only once every 3-4 days and remove solid combustion products, i.e. ash.
Parameters of liquid substances
Liquid materials, like solid ones, are decomposed into the following components: carbon, hydrogen, sulfur, oxygen, nitrogen. The percentage is expressed by weight.
Internal organic ballast of fuel is formed from oxygen and nitrogen; these components do not burn and are included in the composition conditionally. External ballast is formed from moisture and ash.
Gasoline has a high specific heat of combustion. Depending on the brand, it is 43-44 MJ.
Similar indicators of the specific heat of combustion are determined for aviation kerosene - 42.9 MJ. Diesel fuel also falls into the category of leaders in terms of calorific value - 43.4-43.6 MJ.
Since gasoline has more TCT than diesel fuel, it should have higher consumption and efficiency. But diesel fuel is 30-40% more economical than gasoline
Liquid rocket fuel and ethylene glycol are characterized by relatively low TCT values. Alcohol and acetone have the minimum specific heat of combustion. Their performance is significantly lower than that of traditional motor fuel.
Properties of gaseous fuels
Gaseous fuel consists of carbon monoxide, hydrogen, methane, ethane, propane, butane, ethylene, benzene, hydrogen sulfide and other components. These figures are expressed as a percentage by volume.
Hydrogen has the highest heat of combustion. When burned, a kilogram of substance releases 119.83 MJ of heat. But it has a higher degree of explosiveness
Natural gas also has high calorific values.
They are equal to 41-49 MJ per kg. But, for example, pure methane has a higher calorific value - 50 MJ per kg.
Technical requirements for pellets:
| Indicator name | Actual | Compliant |
| Total moisture on working base | 6,89% | ISO 589, DIN 52183 |
| Ash on a dry base | 0,58% | ISO 1171, DIN 51719 |
| Ash on a dry base | 0,54% | |
| Volatile substances on a dry base | 84,33% | ISO 562, DIN 51720 |
| Volatile substances on the working base | 78,52% | |
| Calorific value on dry basis | 4874 Kcal/kg | ISO 1928, DIN 51900 |
| Calorific value per working basis | 4538 Kcal/kg | ISO 1928, DIN 51900 |
| Working calorific value per working basis | 4205 Kcal/kg | ISO 1928, DIN 51900 |
| Sulfur on a dry base | less than 0.01% | ISO 19579, DIN 51724 |
| Sulfur as a working base | less than 0.01% | |
| Hydrogen on dry basis | 6,44% | ISO 12902, DIN CEN/TS 1510 |
| Hydrogen on a working basis | 6,00% | |
| Strength | 97,2% | EN 15210-1 |
| Abrasion | 2,8% | |
| Dust content | +3.15 mm: 98.4% -3.15 mm: 1.6% | CEN/TS 15149-1 |
Classification by variety
“light” (best grade)
“gray” (medium quality)
“dark” (lower quality)
Comparative table of indicators
The table presents the values of the mass specific heat of combustion of liquid, solid, and gaseous fuels.
| Type of fuel | Unit change | Specific heat of combustion | ||
| MJ | kW | kcal | ||
| Firewood: oak, birch, ash, beech, hornbeam | kg | 15 | 4,2 | 2500 |
| Firewood: larch, pine, spruce | kg | 15,5 | 4,3 | 2500 |
| Brown coal | kg | 12,98 | 3,6 | 3100 |
| Coal | kg | 27,00 | 7,5 | 6450 |
| Charcoal | kg | 27,26 | 7,5 | 6510 |
| Anthracite | kg | 28,05 | 7,8 | 6700 |
| Wood pellets | kg | 17,17 | 4,7 | 4110 |
| Straw pellets | kg | 14,51 | 4,0 | 3465 |
| Sunflower pellets | kg | 18,09 | 5,0 | 4320 |
| Sawdust | kg | 8,37 | 2,3 | 2000 |
| Paper | kg | 16,62 | 4,6 | 3970 |
| Vine | kg | 14,00 | 3,9 | 3345 |
| Natural gas | m3 | 33,5 | 9,3 | 8000 |
| Liquefied gas | kg | 45,20 | 12,5 | 10800 |
| Petrol | kg | 44,00 | 12,2 | 10500 |
| Dis. fuel | kg | 43,12 | 11,9 | 10300 |
| Methane | m3 | 50,03 | 13,8 | 11950 |
| Hydrogen | m3 | 120 | 33,2 | 28700 |
| Kerosene | kg | 43.50 | 12 | 10400 |
| Fuel oil | kg | 40,61 | 11,2 | 9700 |
| Oil | kg | 44,00 | 12,2 | 10500 |
| Propane | m3 | 45,57 | 12,6 | 10885 |
| Ethylene | m3 | 48,02 | 13,3 | 11470 |
The table shows that hydrogen has the highest TST indicators of all substances, not just gaseous ones. It belongs to high-energy fuels.
The product of hydrogen combustion is ordinary water. The process does not emit furnace slag, ash, carbon dioxide and carbon dioxide, which makes the substance an environmentally friendly combustible. But it is explosive and has a low density, so this fuel is difficult to liquefy and transport.
European and German pellet quality standards
| Pellet quality standards | Units | DIN Plus | EN plus-A1 | EB plus-A2 | EN-B | DIN 51731 | ONorm M 7135 |
| Pellet diameter | mm | 4-10 | 6(±1) | 6(±1) | 6(±1) | 4-10 | 4-10 |
| Pellet length | mm | ≤5хD | 3.15≤I≤40 | 3.15≤I≤40 | 3.15≤I≤40 | 5 x D1 | <50 |
| Bulk mass of pellets | kg/m3 | — | ≥600 | ≥600 | ≥600 | — | — |
| Heat of combustion of pellets | MJ/kg | ≥18 | ≥16,5 | ≥16,5 | ≥16,0 | ≥18 | 17,5-19,5 |
| Pellet moisture content | % | ≤10 | ≤10 | ≤12 | |||
| Abrasion/Dust | % | ≤ 1 | — | — | |||
| Pellet hardness | % | ≥ 97,7 | ≥ 97,5 | — | — | ||
| Ash content | % | ≤ 0,5 | ≤ 0,7 | ≤ 1,0 | ≤ 3,0 | — | — |
| Ash melting point | °C | — | ≥1200 | ≥1100 | ≥1100 | — | — |
| Chlorine | mg/kg | ≤ 0,02 | ≤ 0,02 | ≤ 0,03 | ≤ 0,03 | — | — |
| Sulfur | mg/kg | ≤ 0,04 | ≤ 0,05 | ≤ 0,05 | ≤ 0,05 | ≤ 0,04 | ≤ 0,08 |
| Nitrogen | mg/kg | ≤ 0,3 | ≤ 0,3 | ≤ 0,5 | ≤ 1,0 | ≤ 0,3 | ≤ 0,3 |
| Lead | mg/kg | — | ≤ 10 | ≤ 10 | ≤ 10 | — | — |
| Chromium | mg/kg | — | ≤ 10 | ≤ 10 | ≤ 10 | — | — |
| Arsenic | mg/kg | — | ≤ 1 | ≤ 1 | ≤ 1 | — | — |
| Cadmium | mg/kg | — | ≤ 10 | ≤ 10 | ≤ 10 | — | — |
| Mercury | mg/kg | — | ≤ 0,1 | ≤ 0,1 | ≤ 0,1 | — | — |
| Copper | mg/kg | — | ≤ 10 | ≤ 10 | ≤ 10 | — | — |
| Nickel | mg/kg | — | ≤ 10 | ≤ 10 | ≤ 10 | — | — |
| Zinc | mg/kg | — | ≤ 100 | ≤ 100 | ≤ 100 | — | — |
We have been producing and selling pellets for more than 5 years. Our pellets have high calorific value and low ash content, High quality, low prices!!!
Conclusions and useful video on the topic
About the calorific value of different types of wood. Comparison of indicators per m3 and kg.
TCT is the most important thermal and operational characteristic of a fuel. This indicator is used in various areas of human activity: heat engines, power plants, industry, home heating and cooking.
Calorific values help to compare different types of fuel according to the degree of energy released, calculate the required mass of fuel, and save on costs.
Do you have anything to add or have questions about the calorific value of different types of fuel? You can leave comments on the publication and participate in discussions - the contact form is in the lower block.